Emergency contraception practices in community pharmacy CPD
Emergency contraception (EC) remains a crucial area where pharmacists can make a real difference to women’s health. Despite advances in access, many women still face barriers—ranging from stigma to uncertainty about where to seek reliable information. With GP appointments no longer required for EC, pharmacists have become the first port of professional consultation for many women, offering both accessibility and trusted expertise. Research shows that 74% of women turn to Google for information about sexual health, highlighting the risk of misinformation and the growing importance of pharmacists as a readily available, evidence-based source of guidance and care. This CPD module will explore the evolving role of the pharmacist in EC management and provide practical insights to support confident, patientcentred consultations.
Hormonal Contraception
Ulipristal acetate (UPA) E.g. ellaOne®
A selective progesterone receptor modulator that can delay ovulation even after the LH surge has begun, offering a wider window of opportunity compared to levonorgestrel-based emergencycontraception. It works by binding to progesterone receptors in the ovary, preventing or postponing the release of an egg and thereby reducing the risk of fertilisation.
Dose and timing
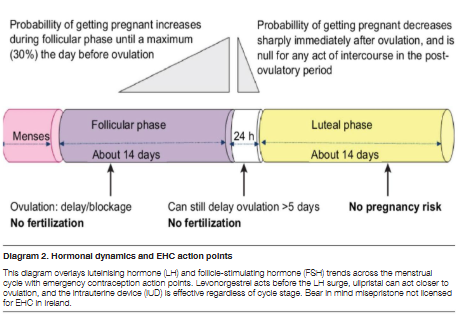
30mg orally, taken as soon as possible after unprotected sexual intercourse (UPSI), and effective for up to 120 hours (5 days) after UPSI. Its ability to delay ovulation even when the luteinising hormone (LH) surge has started makes it a preferred option in later parts of the fertile window.
Effectiveness
Maintains consistently high efficacy throughout the entire 5-day window. Demonstrated to be significantly more effective than levonorgestrel (LNG) between 72–120 hours, particularly when UPSI occurs closer to ovulation. Effectiveness is not reduced by body weight to the same extent as LNG, though some data suggest a modest reduction in women with higher BMI.
Side effects
Headache, nausea, abdominal discomfort, dizziness, and menstrual changes (including earlier or later onset of bleeding). Most side effects are mild and self-limiting.
Special considerations
Breastfeeding should be avoided for 7 days after use, as tiny amounts can pass into breast milk. Hormonal contraception should be delayed for 5 days after taking UPA to avoid reducing its effectiveness. If vomiting occurs within 3 hours of taking the dose, a replacement dose should be taken. Not recommended for women with severe asthma controlled by oral glucocorticoids or those with severe liver disease.
2. Levonorgestrel
Levonorgestrel (LNG) –NorLevo®
Levonorgestrel is a synthetic progestogen that works primarily by inhibiting or delaying ovulation. It does this by suppressing or postponing the luteinising hormone (LH) surge if taken before ovulation has occurred, thereby preventing the release of an egg and reducing the risk of fertilisation. It is ineffective once ovulation has already taken place and does not interrupt or harm an existing pregnancy. Levonorgestrel has been widely used for decades as an emergency contraceptive and is available in single-dose formulations, providing a wellestablished safety profile and predictable pharmacological action.
Dose and timing
The recommended dose is 1.5 mg taken orally as a single dose, ideally within twelve hours of unprotected sexual intercourse (UPSI) to maximise effectiveness. It is licensed for use up to seventy-two hours, or three days, after UPSI, though efficacy decreases with time. If vomiting occurs within three hours of taking the dose, a replacement dose should be taken. It can be taken at any point in the menstrual cycle, provided pregnancy has been reasonably excluded.
Effectiveness
Levonorgestrel is approximately eighty-four percent effective overall when taken within the recommended time frame. Effectiveness is highest when taken within the first twelve hours and declines progressively thereafter. Reduced efficacy is observed in individuals with a body mass index of twenty-six kilograms per square metre or greater, or in those with a body weight above seventy kilograms. In such cases, ulipristal acetate or a copper intrauterine device (Cu-IUD) may be considered more appropriate. It is not effective once ovulation has already occurred.
Side effects
Most side effects if they occur, are mild, short-lived, and resolve without intervention.
Common side effects include nausea (taking the tablet with food or at bedtime may help reduce nausea), headache, dizziness, abdominal discomfort, and fatigue. Menstrual changes may also occur, including earlier or later onset of bleeding, heavier or lighter menstrual flow, and spotting between periods. Temporary breast tenderness or fullness can occur (especially with LNG than UPA) but typically subsides within a few days.
Interactions
Enzyme-inducing drugs reduce efficacy; Cu-IUD preferred in such cases. For Levonorgestrel (LNG), enzyme-inducing drugs that can reduce its efficacy include medicines that increase the activity of liver enzymes (particularly CYP3A4), leading to faster breakdown and lower plasma levels of LNG.
3. Copper intrauterine device (Cu-IUD)
The most effective form of emergency contraception (>99% efficacy). It works by continuously releasing copper ions into the uterus, creating a toxic environment for sperm and eggs, impairing sperm motility and viability, and preventing fertilisation. If fertilisation has already occurred, it can also inhibit implantation.
Timing
Can be inserted up to 5 days after UPSI or up to 5 days after the earliest possible date of ovulation. Can be used at any time in the menstrual cycle, provided pregnancy has been reasonably excluded.
Advantages
Offers long-term contraception lasting 5–10 years, depending on device type. Completely hormone-free, suitable for women who cannot use oestrogen or progestogen. Provides ongoing protection without daily adherence. Can be removed at any time if pregnancy is desired.
Side effects
May cause heavier or more painful periods in the first few months after insertion, which usually improves with time Initial cramping or discomfort at insertion is common but typically shortlived. Rare risks include uterine perforation, expulsion, and pelvic infection (mainly in the first 20 days after insertion).
Diagram 1. Mechanism of action of LNG and UPA

Repeat use and safety of emergency contraception.
Repeating use in the same cycle is safe but may disrupt cycles. UPA and LNG should not be taken together for the same UPSI. Pregnancy test if menstrual cycle (period) period is over 7 days late. Factors affecting choice of emergency contraceptive
Factors affecting choice of Emergency Contraception
Overall safety profile of LNG and UPA
Both LNG and UPA have extensive safety data supporting their use, including in adolescents, and breastfeeding women (with some restrictions for UPA described above). Neither is associated with serious long-term adverse effects. Studies have shown no evidence of teratogenicity if EHC is inadvertently taken during an early pregnancy. Importantly, these medicines do not cause abortion and will not affect an established pregnancy. They act before implantation, primarily by delaying ovulation.
Issues with regular use of EHC
EHC, primarily levonorgestrel (LNG) and ulipristal acetate (UPA) is designed for occasional, urgent use after unprotected sexual intercourse (UPSI) or contraceptive failure. While both are safe and effective when used appropriately, regular reliance on EHC instead of consistent contraception can lead to a range of clinical, practical, and psychosocial issues including:
Reduced overall contraceptive effectiveness
EHC is less effective than most regular contraceptive methods. LNG reduces pregnancy risk by around 84% when taken within 72 hours of UPSI, while UPA is more effective but still not as dependable as long-acting reversible contraception (LARC) or combined oral contraceptives.
Menstrual cycle disruption
Repeated EHC use in a short period can cause unpredictable menstrual changes including shortened or lengthened cycles; lighter or heavier bleeding; intermenstrual spotting. These are not medically harmful but can cause anxiety and complicate pregnancy detection.
Hormonal side effects
The side effects discussed already of occasional EHC use are usually mild, but frequent exposure can increase the likelihood of side effects including side effects becoming more persistent and increased intensity the more EHC is used.
Impact on regular hormonal contraception
Using UPA regularly may interfere with the efficacy of ongoing hormonal contraception. After UPA, there must be a 5-day delay before restarting hormonal contraception, during which barrier methods must be used. Repeated courses can therefore lead to prolonged contraceptive gaps without full contraceptive protection thus actually increasing chance pregnancy longer term.
Psychological and emotional effects
Some patients report feeling anxious, guilty, or judged when accessing EHC multiple times. This can impact selfesteem and decision-making. Pharmacists play a key role in non-judgmental support and reframing consultations towards empowerment and choice.
Pharmacist counselling points for EHC
Some of the points below for counselling are mentioned already but are simply repeated to give coherent counselling advice in one section.

Timing is critical
EHC should be taken as soon as possible after unprotected sexual intercourse (UPSI). LNG is licensed
• Breastfeeding: LNG is safe; UPA requires a 7-day breastfeeding break.
Administration and missed doses
Take one tablet orally with water. If vomiting occurs within 3 hours, repeat the dose. Advise patients to avoid alcohol or excessive caffeine if feeling nauseated, although no direct interaction exists.
Expected side effects
As detailed above; most get no side effects, if they occur, they are generally mild and very short term
Contraceptive protection afterwards
• After LNG: continue or start hormonal contraception immediately; use condoms for 7 days (9 days for Qlaira® users).
• After UPA: delay hormonal contraception for 5 days, then use condoms until hormonal method has been active for 7 days (or 9 days for Qlaira®).
• Emphasise that EHC does not protect against pregnancy for further UPSI later in the same cycle.
Pregnancy testing and follow-up
Advise pregnancy test if:
• Menstrual cycle (period) is more than 7 days late.
• The next period is unusually light or heavy.
• There are pregnancy symptoms (nausea, breast tenderness, fatigue).
STI awareness and referral
• EHC does not protect against sexually transmitted infections (STIs).
• Offer STI screening referral where indicated.
• Encourage condom use for both infection and pregnancy prevention.
Confidentiality and supportive environment
• Provide EHC in a private consultation area.
• Use a non-judgmental, supportive tone, especially for younger patients.
• Reassure that access to EHC is legal and confidential.
Long-term contraception advice
• Frequent EHC use is less effective and more disruptive than regular contraception (e.g., the pill).
• Discuss options such as oral contraceptives, implants, injections, or IUDs
Special circumstances
• Enzyme-inducing drugs (e.g., rifampicin, carbamazepine) reduce efficacy – recommend copper IUD or double LNG dose (off-label).
• Postpartum women can use LNG; UPA not advised during breastfeeding without temporary milk cessation.
• Perimenopausal women still require contraception until menopause confirmed.
Supply considerations of emergency hormonal contraception (EHC) in Ireland –shortages and alternatives Guidance for pharmacists on supply considerations, if shortages occur, is outlined below.
1) Current supply issues & expected end dates
• The HPRA maintains the official national shortages list. As of August 2025, Ulipristal® acetate (UPA 30 mg) had a shortage earlier in 2025 (Rowa brand only) with Ella One® always available. Levonorgestrel (LNG 1.5 mg) has not had persistent shortages recently, but both remain subject to intermittent availability issues.
• Always check the HPRA shortages portal and IPU/NHPC updates for real-time information.
2) Use of unlicensed (ULM/EMP) versions
• If licensed brands are unavailable, supply of unlicensed emergency contraception is only permitted through the Exempt Medicinal Product (EMP) framework. This requires consultant initiation, patient informed consent, and invoice evidence.
• Claims must be processed under HSE Pharmacy Circular 12/23 (EMPs).
3) Free contraception scheme and coverage
• The Free Contraception Scheme covers licensed emergency contraception supplied via pharmacy for eligible patients (17th birthday to the day before 36th birthday).
• If the product is unlicensed, it cannot be supplied under the pharmacy OTC EHC arm of the scheme. Instead, EMP rules apply (prescription + consultant initiation).
4) PCRS claiming & 777 Codes
• For DPS/LTI/HAA schemes, unlicensed medicines are claimed using 777xx codes with invoice evidence attached.
• 77750 = 0% VAT, 77752 = 23% VAT (confirm exact suffix with PCRS before submission). Oral products have zero VAT.
• These codes are not used for standard Free Contraception Scheme supplies of licensed products.
5) Workflow for pharmacy teams
1. Check HPRA/IPU shortages list and wholesalers for alternative licensed brands.
2. If UPA is unavailable, consider LNG (if ≤72 hours) or refer for copper IUD.
3. If no licensed option is obtainable, request prescriber/ consultant initiation for EMP supply.
4. For EMPs, process claims via 777xx codes with invoices (DPS/ LTI/HAA) or GMS list codes where available.
5. For licensed EHC supplied without prescription under the Free Contraception Scheme, claim usual fee plus ¤11.50 service fee.
6. There was never any ULMs of EHC needed yet in Ireland
Despite my description of protocols if ULMs of EHC are required, actually there has never thankfully been a need for a ULM version EHC needed or used in Ireland as there was always an alternative licensed version; both levonorgestrel (Norlevo®) and ulipristal acetate (Ella One®) have an alternative generic version (both Rowa). Rowa generic was temporarily short in 2025, but alternative licensed brand versions were available, unlike HRT where ULMs are often required.
Free emergency hormonal contraception HSE scheme from pharmacies
Free emergency hormonal contraception is available anytime from participating pharmacies for women aged 17 to 35 under the HSE scheme. It is also free outside this age group if patients have a medical card arranged directly in the pharmacy without needing to visit your GP. Patients not within the 17-to-35-year age group or without a medical card are still entitled to EHC via pharmacy but they will have to pay the price of the product (price varies by pharmacy).
All pharmacies have a private consultation room to enable a discreet, confidential, and confidential service. The pharmacist will ask health questions (many pharmacies have a confidential health questionnaire) to ensure it is safe and to choose the right one for the patient based on criteria given already. The process takes only minutes, making it quick, discreet, and effective in preventing unintended pregnancies through timely access.
Providing EHC free of charge at pharmacies removes a major financial barrier. A single course of EHC can cost ¤15–¤35 privately, which may deter some women from seeking it promptly. Timeliness is critical.
Aged 16 and below
Pharmacists should also be aware that the age of sexual consent in Ireland is 17 years. Where appropriate, pharmacists need to assure themselves of the age of the patient. Having regard to the age and circumstances of the individual patient, and any child protection issues arising, pharmacists should consider whether referral to a medical practitioner, other healthcare professional, or other agency or authority is appropriate
Pharmacists are legally allowed to supply EHC without parental consent to those aged 16 years and over. Individuals in this age group are considered capable of giving their own consent for medical treatment. There is no strict age limit for the product itself.
Under 16 Years Old
If someone is under 16, pharmacists should seek parental or guardian consent before providing EHC or they may decide it is best for the patient to see their GP depending on circumstance and the pharmacist’s professional opinion.

Written by Eamonn Brady, MPSI
Eamonn is the Supervising Pharmacist and Owner of Whelehan’s Pharmacy’s, Pearse Street and Clonmore, Mullingar
Catch more at IPN HERE
Read IPN September HERE










